
The pharmacovigilance agreements (PVA) landscape has evolved over recent decades with rapid growth in the number and complexity of partnerships, mergers, and acquisitions between pharmaceutical companies. Simultaneously there has been increasing scrutiny from regulatory authorities. Detailed regulations and guidance are lacking in this domain; hence companies have developed their own processes, templates, and tools, which have headed in different directions. Where feasible, marketing authorization holders (MAHs) have written contracts based on mutually understood requirements. Currently, MAHs are striving to find optimal solutions that safeguard patients, and in turn, support pharmacovigilance compliance. Through the TransCelerate BioPharma consortium MAHs are seeking simplification and efficiencies, to optimize the process of developing contractual agreements for pharmacovigilance. A survey of MAHs confirmed the perceptions above, and the need for efficient solutions to help navigate through the maze of complexity. The authors have led the development of tools and techniques to enable partnership between MAHs, and ultimately to support patient safety.


Avoid common mistakes on your manuscript.
Pharmacovigilance agreements (PVA) and alliance partnership management is an area of constant challenge across Pharmacovigilance (PV) departments. The landscape is continuously evolving, with an ever-increasing number and complexity of partnerships throughout the industry. In the past, companies predominantly managed the development of medicinal products in-house. However, in today`s environment with increased numbers of mergers and acquisitions there are agreements which can consist of multiple parties and involve complex regulatory operating models. Collaboration is required at different stages of the product lifecycle from early development to post-marketing, and each phase poses different challenges. Industry has expanded over a larger network of vendors and wider range of medicinal products, devices (medical devices, software as a medical device), and combination therapies, but also advanced therapy medicinal products (ATMP), vaccines, radioligand, cell and gene therapies, etc. The need for rapid development of effective therapies for SARS-CoV-2 manifested in reduced development timelines along with the need for fast negotiations and approvals of PVAs across companies.
In addition, there is an increase in the scrutiny by regulatory authorities with the incremental expectation of both pharmacovigilance compliance and of regular, risk-based auditing and inspecting. There is not only an expectation but also a demand from all parties impacted by PV activities, and specifically regulatory authorities, to ensure compliance with all operational- and process-based regulations. There is a regulatory requirement for companies to establish a PVA if they are collaborating with other companies on post-marketed or clinical compounds [GVP VI.B.7/C.1.2/C.2.2]. Although regulations are available for specific PV activities (see specimen list which is not intended to be all-inclusive [1,2,3,4,5,6,7,8,9,10]), there is no overall regulation or guidance for a PVA template, nor are there other requirements that addresses this topic.
As companies set up partnerships, many aspects of the PVA must be negotiated and agreed upon to be able to operationalize and meet regulatory requirements for pharmacovigilance. Consequently, companies have developed their own internal processes, templates, and tools to manage PVAs. Such tools and processes can vary immensely across companies and may lead to misalignments and often convoluted or prolonged negotiations. Furthermore, there is a risk of a disconnect between information in the PVA and the operationalization of the agreement’s requirements. These challenges may delay the timely start of clinical trials and/or marketing authorizations of products and potentially influence patient safety and choice of treatment by healthcare providers.
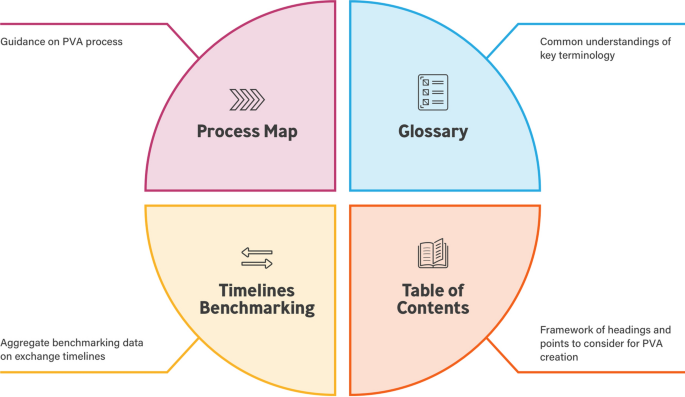
There is a need for simplification and streamlining of structured processes and content that can be used by companies of differing sizes and varied portfolios when creating PVAs. The TransCelerate PV Agreements Optimization (PVAO) Initiative was established to develop tools to optimize PVA processes and create efficiency for all parties. This initiative focused on addressing four challenges for PVA development: (1) Process Map, (2) Table of Contents (ToC), (3) Glossary/definitions, and (4) Timelines for Safety Data Exchange. An integrated set of solutions was developed to address these challenges. This paper provides an overview of the first three solutions and their development. Footnote 1
This project involved large to medium-sized pharmaceutical companies from the TransCelerate BioPharma consortium that comprises 20 member companies. In order to gain a preliminary understanding of the PVA landscape, an initial list of five questions (Table 1) was prepared and distributed to the companies working on the PVAO Initiative. Anonymized and aggregated results were used to develop the integrated solutions.
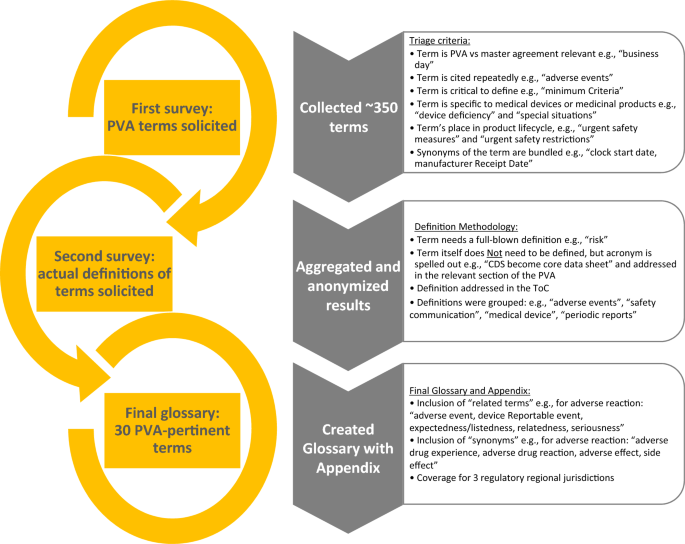
A second survey was used to solicit definitions for the terms. Results were aggregated and anonymized, and once the most common PVA terms were identified, draft definitions were developed using a collegial review and evaluation by the glossary team. The team determined if (1) a definition qualified for the incorporation into the glossary section in the PVA; (2) if a simple naming of the term (or acronym) was sufficient (e.g., core data sheet, periodic safety update report) in the body of the PVA; and/or (3) the definition would be addressed in the ToC. Definitions were then grouped by categories (e.g., adverse events [AEs], safety communication, medical device, and periodic reports).
The development of the glossary included the consideration of regulations in the US, EU, and Japan including ICH or, in the absence of regulatory language, definitions were developed based on a combination of text from individual companies, the team’s subject matter expertise, and on the team’s collaborative research. During this exercise, it was determined that other terms that were similar in scope and/or may be helpful to provide additional context for the user would be collectively referred to as “associated terms” in the glossary.
A literature search was performed using Ovid Databases: MEDLINE(R) ALL 1946 to May 03, 2021 and Embase 1974 to May 03, 2021. The following key words were searched: Pharmacovigilance contract, Pharmacovigilance agreement, Vigilance agreement, Vigilance contract, Safety data exchange, Pharmacovigilance licensing, Pharmacovigilance co-development/co-marketing agreement, PVA lifecycle, PV alliance, PV obligations, Pharmacovigilance partnership, and Pharmacovigilance contract framework.
PVAs follow a lifecycle beginning with the establishment of a new partner collaboration and ending with the termination of PV responsibilities in that partnership. Based on such lifecycle a flexible and nimble framework “process map” (Fig. 2) was developed and published in the online version with embedded tools and helpful considerations [12].
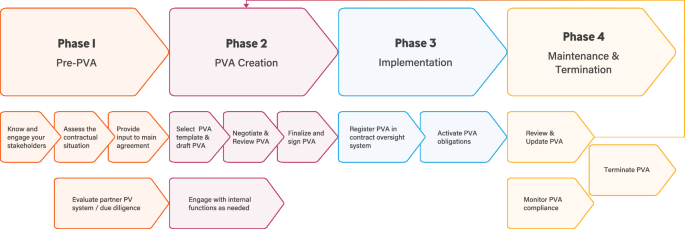
The process map provides a phased approach to the overall PVA process and allows companies to follow a pathway through the maze and identify potential blind alleys, cul-de-sacs, or dead ends. The four sequential phases: (1) Pre-PVA, (2) PVA creation, (3) PVA implementation, and (4) PVA maintenance and termination were supported with helpful considerations that foster efficiency and compliance in PVA-related activities.
While developing the process map, the team highlighted potential areas for improvements (Table 2) and developed tools to help optimize PVA business operations and simplify the overall process. These tools provide detailed, tangible suggestions or considerations for improving efficiency. The tools are embedded in the online version of Fig. 2 [12]. All tools may be downloaded and customized by the user. One example is the “Systematic Approach to the PVA Negotiation” tool for structuring the PVA negotiation process by using project management elements. The tool offers contract timelines planning, agreement on PV collaboration principles, and appropriate internal company stakeholder engagement, which may facilitate faster alignment. The “Periodic PVA Review Checklist and Documentation” tool helps facilitate a streamlined PVA review process. The “Pre-PVA Involvement of Pharmacovigilance (PV) Function” tool describes how to build internal communication with functions that are entering into new partnerships.
The diagram (Fig. 3) provides a summary of the results.
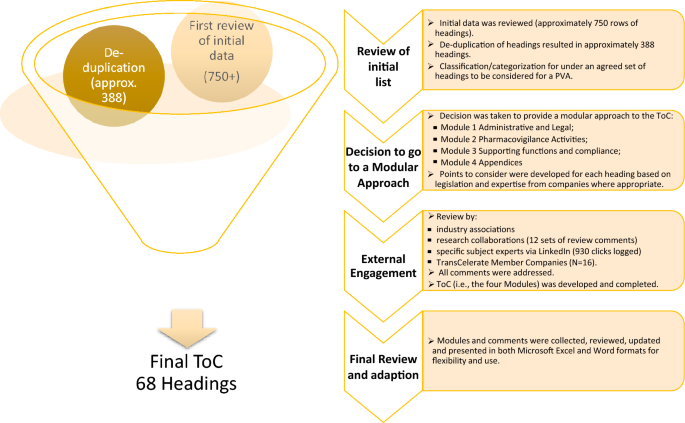
The final format of the ToC is modular consisting of four modules:
Each module comprises of a series of headings, some of which may have sub-headings. Each heading, or sub-heading, has a series of points to consider.
Due to the diversity of global regulations, the ToC does not include reference to PV regulations from all possible countries. Thus, the ICH regions (Europe, US, and Japan) were the points of focus. However, the document is written in such a way to make it useful in single countries, via addressing the applicable national regulatory requirements. With respect to the types of contracts, again allowing for flexibility, consideration was given to both pre-authorization (clinical trials) and the post-marketing setting. Depending on the type of agreement in such areas—in-licensing, out-licensing, distribution agreements, clinical trial supply agreements and others, the relevant headings and PtC can be used flexibly according to the needs of the parties. In addition, annexes outlining PtC for combination products and vaccines are included in the ToC.
In developing the ToC, the team adopted headings and a straightforward modular approach was developed to group similar topics and areas in the PVA together.
The intent was to provide a simple structure for companies, auditors, and inspectors.
An example of Points to Consider is provided on Fig. 4 and a list of the Key Headings for the ToC is provided in Appendix 3.
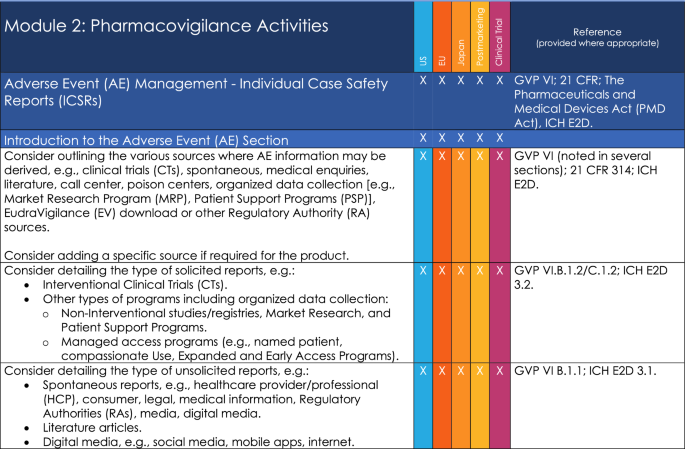
One company’s PVA terms often reflect the specific terms that are used throughout that particular company. Internal alignment for operationalization within one company may not necessarily align with the partner’s terms potentially resulting in circular discussions and extended negotiations. A large number of terms were returned in the responses to the survey of member companies. Several terms were specific to individual companies and many of the terms were considered by the team not to be essential for a PVA. In addition, the same words were being used for similar terms. A single harmonized glossary providing unified definitions enables companies to align more easily with the basic principles within the PVA, and may facilitate simultaneous implementation by the partner companies.
The glossary provides definitions for 30 key PVA terms. The glossary may, therefore, be particularly helpful when a PVA is required in a short timeframe as negotiating parties may elect to utilize the definitions so that more time can be allocated to negotiations on other important parts of the PVA, e.g., timelines. The member survey results also highlighted variations in how glossary terms are captured in PVAs: not all companies include a glossary/list of definitions, and the number of terms varies. The glossary is intended to be flexible for mature or novel PVA development processes and could potentially reduce PVA negotiation time.
In addition to the terms and definitions in the glossary, supporting documentation was developed as part of the solution and compiled into an addendum. The addendum provides comprehensive information about the references that were used to support the creation of the Glossary definitions. Furthermore, it provides cross-referencing of terms through hyperlinks that are integrated in the document, a listing of synonymous terms where applicable, and associated terms, which may provide additional context to the user.
The Process Map, Table of Contents, and Glossary Solutions are made available in a web-based interactive toolbox [12]. A fourth solution (Timelines Benchmarking) remains under development at the time of this paper. All solutions were designed to complement each other, and several can be downloaded and customized according to the needs of each legal entity (Fig. 5) Footnote 3 .
Specific challenges were related to a variety of scenarios as noted in the introduction including an example of small biopharmaceutical companies working across territories. The challenges inherent here are not limited to the joint working of small and large companies, but also related to the limited experience of third countries with other territory regulation and requirements placed concerning PVAs.
Increased complexity of PVAs and limited information in both the public domain and regulations prompted a desire for more efficient approaches. The member companies moved through a PVA maze by collecting and analyzing the information from the work streams as described in the results section.
Several different questions arose, including:
“What is defined as a PV Agreement?”
“What types of contractual relationships require a PVA?”
“Are PVAs only required for the full sharing of commercial sales between two MAHs?”
“Does an agreement with a vendor providing outsourcing activities require a PVA?”
“Does an agreement with a contract research organization (CRO) for clinical trial supplies require a PVA?”
“Should clauses covering pharmacovigilance responsibilities be located in the main agreement or in an annex specific for pharmacovigilance?”
And finally, “Should we refer to a PVA or safety data exchange agreement (SDEA)—are they the same?”.
Our research showed that most problems were encountered when negotiating the details of PVAs. Even after PVA execution, further difficulties are noted during the operationalization of the contractual commitments due in some measure to the variety and complexity of agreements required multiple customizations of existing in-house processes.
Literature research was conducted which confirmed that there was little information regarding the PVA process in the public domain. Most of the relevant publications were dated before 2013, with the majority published between 2000 and 2010 [13,14,15,16,17,18]. Although some information remains applicable to today`s PV systems, the complexity described above was not present, hence it was not considered.
Sixteen TransCelerate Biopharma member companies (with additional input of stakeholder engagement) attempted to find potential efficiencies. The current complex PVA landscape is managed across differing company frameworks that often allow agreement on the general principles but may sometimes lead to disagreement and contentious discussions concerning the details. The initiative supported a healthy and constructive discussion on the most ambiguous areas in the PVA negotiation and challenging types of PVAs. As a result, solutions offering a flexible and agile framework were proposed in the form of a PVAO Suite containing a process map, table of contents, glossary and timelines benchmarking to support the facilitation of earlier issue resolution and more efficient collaboration between the parties negotiating the PVA.
The management of PVAs requires time, resources, and expertise to maintain regulatory compliance. A goal was to illustrate the PVA lifecycle and provide considerations and tools to support organizations that engage in PVA activities to simplify, optimize, and improve efficiencies in the PVA process.
The PVA journey travels through a maze of different parties, including internal and external stakeholders, various geographic regions, associated regulations, and product variations. All of the above are further complicated by the existence of fundamental operational differences between pharmaceutical companies. Negotiations often involve circular discussions with the revisiting of different elements within the agreement process and iteration until the goal of a signed contract is achieved. By adoption of the process map, organizations may be better able to navigate their way through the required steps and reach the desired goal without experiencing repeated delays.
Embedded tools within the Process Map intend to address potential areas for improvements (Table 2) and to help optimize PVA management. Companies may have multiple functions that are entering into new partnerships or due diligence matters and building sustainable internal communication between PV and these functions may become key to success for early involvement of PV. The tool “Pre-PVA Involvement of Pharmacovigilance (PV) Function” offers support to organizations in fostering internal communication. Bottlenecks along the PVA lifecycle can be managed by implementing structured processes. The “Periodic PVA Review Checklist and Documentation” tool suggests how to streamline PVA review process, whereas “Systematic Approach to the PVA Negotiation” tool proposes structuring the PVA negotiation process by using project management elements. These tools provide tangible process descriptions that may be valuable guidance for improving efficiency and support compliance.
At times, during negotiations or even during the operationalization of the PVA, partners may experience misalignment and potentially compliance issues over the meaning of a term and its implications for the success of the implementation of the PVA. Defining PVA terms that are meaningful and unambiguous solidifies the respective parties’ comprehension rather than allowing them to meander toward dead ends. The value of offering commonly understood definitions for terms such as “awareness date,” “minimum criteria,” “day zero,” “invalid,” or “incomplete case” can result in greater agreement clarity, clearer expectations, and may help reduce short- and long-term operational hurdles.
A goal of this project was to develop a comprehensive and flexible framework for managing PVAs that may help to reduce the time taken for PVA development and support compliance with regulations and to the PVA. Two partner companies using information from the ToC or starting negotiations with the same understanding of the PVA definitions can potentially ultimately reduce the negotiation time, which could allow the focus to be spent on specific topics of relevance. Moreover, a transparent and documented end-to-end process has the possibility to avoid PVA process issues and faster resolution thereof.
These tools aim to enhance efficiency in PVA development and simplify processes which could then ultimately support the training of personnel and conduct of audits/inspections. The structure and processes offered by these tools may provide a more time-efficient means of ensuring that appropriate auditing is feasible for all partners, and could ultimately reduce the number of findings and observations.
All stakeholders are striving for regulatory compliance, ideally accompanied by efficiency gains, operational excellence, and achieved in a collaborative manner. In the future, such tools may help contractual partners to achieve these goals.
The PVAO suite was designed to support greater internal streamlining of the process, enabling and providing further opportunities. The authors noted that there are other issues in the PVA area for further investigation, which are outside the remit of the current project. It is considered that PVAO solutions provide a foundational platform that could further expand into areas such as, but not limited to, automation of the PVA development process and model contracting.
An important area of the PVA lifecycle is the monitoring of PVA obligations during the maintenance phase of the PVA lifecycle, as this allows early identification of potential non-compliance. A strong monitoring program can inform the risk-based audit strategy. Companies struggle with defining the level of granularity when setting up the monitoring processes, but also with the lack of guidance or the availability of methods and tools.
Audits and inspections may benefit from the use of the PVAO suite in terms of transparency of the process with a recognizable structure and framework for the PVAs. Audits and inspections are a challenge in terms of time, resources, and findings and the use of such tools may help to reduce such challenges.
There is a global increase in the number of medical device regulations [19,20,21,22,23,24,25,26,27,28,29]. As a result, the development of device vigilance agreements is increasingly challenging for many companies. The need for more guidance and solutions is manifest. Overall, the use of such tools may potentially reduce the time spent on drafting and negotiating PVAs and enable a concentrated focus on specific topics of importance.
In order to navigate effectively through the vigilance agreements maze, there is a need for simplification and process streamlining to facilitate regulatory compliance, and meet auditors’ and inspectors’ expectations. The TransCelerate PVAO Initiative was established to support this effort in relation to PVAs. The pharmaceutical industry has expanded to encompass a network of vendors, partners, and a wider range of medicinal products, devices, and combination therapies.
TransCelerate’s PVAO suite of solutions aims to support more efficient and effective collaboration between partners and provides a step forward in rationalizing a complex area. Faster and more efficient management of PVAs can potentially help to mitigate delays to patient access because quicker PVA execution may support earlier clinical trial start, managed access programs, product launch, or asset transfer activities. This suite of tools is not a one-size-fits-all solution; rather, it lays a foundation that can be further elaborated upon and expanded to cover other elements of the PVA business, such as devices and model contracting, and obligation monitoring. Further research, outside the remit of the current project, could be to embrace technology and digitalization advancements. It is clear that if automated processes are an option for the creation and management of PVAs, there is a genuine possibility that artificial intelligence (AI) can aid governance of PV systems.
Our aim is to suggest new, efficient ways of working, to simplify navigation through to completion of new PVA partnerships. We recognize that there is a need to provide agile solutions based on a nimble framework and accompanied by flexible tools. Fostering better and more effective collaboration between companies can result in improved compliance and efficiency that helps to support patient safety and potentially facilitates earlier access to medicines.
Inspection findings were not only based on anecdotal and experiential evidence, but from Regulatory Authority websites including MHRA, TGA, BfArM, and FDA.
Note: Timelines benchmarking is currently under development at time of writing.The authors gratefully acknowledge the support of the following individuals for consultation, support and review: Mayur Patel, Yvonne M. Gibble, Elizabeth MacEntee Pileggi, Wendy Manko Singer, Kim Kidd, Anna Carina Wiborg Simonsen and Jillian Horvath. We would also like to thank the team members of the Pharmacovigilance Agreements Optimization (PVAO) Initiative solutions that are discussed in this article as well as the TransCelerate organization: Dibab Abera, Milton Apotsos, Anna-Maria Bornemann, Ralph Bellocco, Caroline Densmore, John Dobbins, Jeneen Donadeo, Carole Ann Gerhart, Denissa Gordy, Justin Joubert, Cynthia Anne Kelley, Lisa Kennell, Emily Liffick, Valentina Mancini, Sabura Matthews, Leigh Anne Minnier, Alvaro Moreno, Melinda S Moriarty, Ashley Pachter, Michelle Parsons, Jonathan Rowell, Sarah Sahin, Zuleika Sanchez, Robin Schutz, Isabelle Schnitzler, Marion Schubert, Ragin Shah, Willemijn Van der Spuij, Hal Ward, and Jennifer Whittaker.
Funded by TransCelerate Biopharma, Inc.